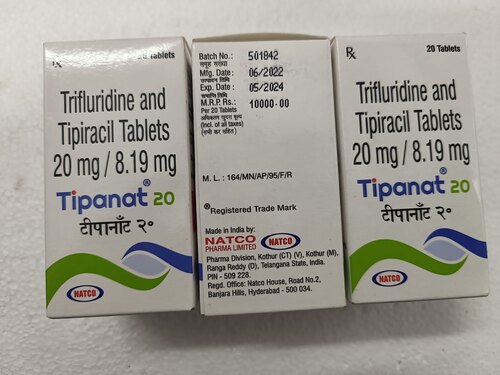
Trifluridine And Tipiracil Tablets
Product Details:
- Dosage Form Film-coated tablet
- Indication Metastatic colorectal cancer (previously treated) & gastric cancer
- Salt Composition Trifluridine 15 mg + Tipiracil 6.14 mg
- Enzyme Types Thymidine phosphorylase inhibitor (Tipiracil), Nucleoside analog (Trifluridine)
- Feature
- Ingredients
- Application
- Click to View more
Trifluridine And Tipiracil Tablets Price And Quantity
- 5 Bottle
- 8500 INR/Bottle
Trifluridine And Tipiracil Tablets Product Specifications
- 24 months from manufacturing date
- Film-coated tablet
- Not applicable (no fermentation required)
- Trifluridine 15 mg + Tipiracil 6.14 mg
- Thymidine phosphorylase inhibitor (Tipiracil), Nucleoside analog (Trifluridine)
- Store below 30C in a dry place, keep in original packaging
- Metastatic colorectal cancer (previously treated) & gastric cancer
- Odorless
- Inhibits thymidine metabolism, increases trifluridine bioavailability
- White to off-white, round, biconvex film-coated tablet
- Not applicable (synthetic pharmaceutical tablets)
Trifluridine And Tipiracil Tablets Trade Information
- 1000 Bottle Per Week
- 4 Days
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- All India, South India, Central India, West India, North India, East India, Gujarat, Karnataka, Kerala, Lakshadweep, Mizoram, Meghalaya, Manipur, Andhra Pradesh, Bihar, Chandigarh, Daman and Diu, Goa, Jharkhand, Odisha, Punjab, Assam, Delhi, Dadra and Nagar Haveli, Andaman and Nicobar Islands, Arunachal Pradesh, Chhattisgarh, Haryana, Himachal Pradesh, Jammu and Kashmir, Madhya Pradesh, Maharashtra, Nagaland, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, Pondicherry, Uttar Pradesh, Uttarakhand, West Bengal
Product Description
Trifluridine and tipiracil tablets, typically available in the strength of 20mg trifluridine and 8.19mg tipiracil, are used in the treatment of certain types of advanced or metastatic cancers. Here's an overview of its use:
1. Colorectal Cancer Trifluridine and tipiracil tablets are approved for the treatment of metastatic colorectal cancer (CRC) that has progressed after standard chemotherapy and other treatments. It is specifically indicated for patients who have previously received fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, anti-VEGF therapy, and, if RAS wild-type, an anti-EGFR therapy.
2. Gastric or Gastroesophageal Junction (GEJ) Cancer Trifluridine and tipiracil tablets may also be used in the treatment of advanced gastric (stomach) or gastroesophageal junction (GEJ) cancer in patients who have previously received at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, and either a taxane or irinotecan.
Trifluridine is a nucleoside analog that interferes with the synthesis of DNA, which is necessary for cancer cell replication. Tipiracil is a thymidine phosphorylase inhibitor that helps maintain effective trifluridine levels in the bloodstream by inhibiting its degradation. The combination of trifluridine and tipiracil allows for improved delivery and retention of trifluridine within cancer cells, enhancing its anti-cancer effects.
These tablets are typically taken orally as directed by a healthcare professional. The dosage and treatment regimen may vary depending on factors such as the type and stage of cancer, overall health, and individual response to treatment. Common side effects may include fatigue, nausea, vomiting, diarrhea, decreased appetite, and low blood cell counts.
It's important for patients to be monitored closely by their healthcare provider while taking trifluridine and tipiracil tablets to manage any side effects and ensure the treatment remains effective. As with any cancer treatment, it's crucial to discuss the potential benefits and risks with a healthcare professional to make informed decisions about treatment options.
How Trifluridine and Tipiracil Tablets Work
This medication combines a nucleoside analog (trifluridine) and a thymidine phosphorylase inhibitor (tipiracil). Together, they disrupt the growth of cancer cells by inhibiting the metabolism of thymidine, an essential component for DNA synthesis. Tipiracil also increases the concentration of trifluridine, ensuring more effective cytotoxic action against cancerous cells.
Usage and Administration Guidelines
Trifluridine and Tipiracil Tablets are taken by mouth as prescribed by an oncologist. Adherence to the dosing schedule is crucial for optimal efficacy. Dosage is determined by body surface area and treatment cycle. Tablets should be swallowed whole with water, without crushing or chewing. They are marked for identification and available in blister-strips inside protective box packaging.
Safety, Precaution, and Storage Information
Use of these tablets requires monitoring for myelosuppression and is not advised for individuals with severe liver or kidney impairment. Particular caution is advised during pregnancy (Category D, positive risk evidence). Always store the medication below 30C in a dry environment, away from direct sunlight, and keep the tablets in their original packaging to maintain stability and efficacy.
FAQ's of Trifluridine And Tipiracil Tablets:
Q: How should Trifluridine and Tipiracil Tablets be administered?
A: These tablets are taken orally, usually twice daily, as directed by your prescribing oncologist. They should be swallowed whole with water and not chewed or crushed. Maintain the prescribed schedule and always consume the tablets with or shortly after food to improve absorption.Q: What conditions are Trifluridine and Tipiracil Tablets used to treat?
A: This medication is indicated for the treatment of adults with metastatic colorectal cancer and gastric cancer who have previously been treated with standard chemotherapy regimens.Q: When should I avoid taking Trifluridine and Tipiracil Tablets?
A: You must not use this medication if you are allergic to trifluridine, tipiracil, or any of the listed excipients. It is also contraindicated in pregnancy due to its Category D risk and is not recommended if you have severe hepatic or renal impairment.Q: Where should Trifluridine and Tipiracil Tablets be stored?
A: Store the tablets below 30C, in a dry location away from moisture, and always in the original blister packaging to protect their quality. Keep the medication out of reach of children.Q: What is the process for monitoring therapy with these tablets?
A: Patients on this chemotherapy must undergo regular blood tests to monitor for myelosuppression (suppression of bone marrow activity), which may necessitate dosage adjustments or interruptions as advised by a healthcare provider.Q: How do these tablets benefit patients with metastatic cancer?
A: Trifluridine and Tipiracil Tablets offer an oral chemotherapy option that can be administered in outpatient settings. They have demonstrated enhanced cytotoxic activity, helping to control the progression of disease in patients who have limited treatment alternatives.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+



 Contact Us
Contact Us Call Me Free
Call Me Free
