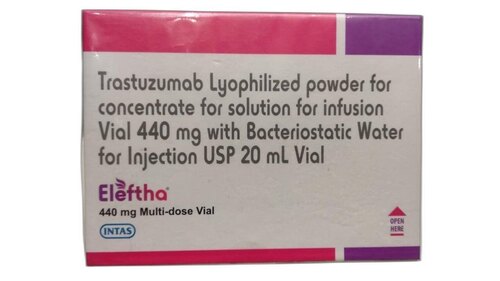
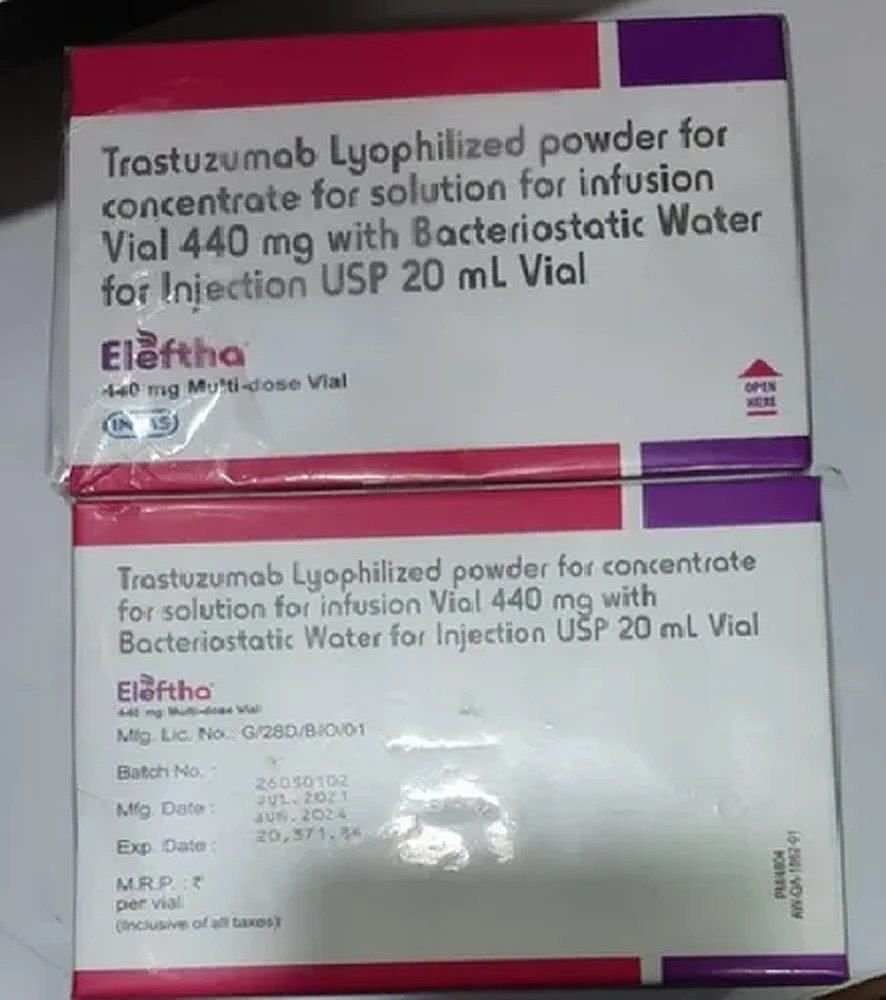
ELEFTHA 440MG
Product Details:
- Salt Composition Alpha-Amylase 440mg
- Dosage Form Powder
- Indication Boosts starch conversion in alcohol fermentation, suitable for industrial brewery, distillery, ethanol, and sugar processes
- Enzyme Types Alpha-Amylase (endoamylase)
- Feature Thermostable amylase enables reliable starch breakdown; enhances alcohol yield; high purity; suited for continuous fermentation
- Ingredients Alpha-Amylase Enzyme Extract, Food-Grade Stabilizers
- Application Alcohol production, Brewery, Distillery, Ethanol manufacturing, Sugar processing
- Click to View more
ELEFTHA 440MG Price And Quantity
- 8000.0 INR/Number
- 1 Number
ELEFTHA 440MG Product Specifications
- Optimal temperature 60C 70C
- Alpha-Amylase Enzyme Extract, Food-Grade Stabilizers
- Boosts starch conversion in alcohol fermentation, suitable for industrial brewery, distillery, ethanol, and sugar processes
- Powder
- Alcohol production, Brewery, Distillery, Ethanol manufacturing, Sugar processing
- 24 months
- Alpha-Amylase (endoamylase)
- Store in a cool, dry place below 25C, tightly sealed
- Optimal pH 5.5 - 6.5
- Alpha-Amylase 440mg
- 440mg activity (high potency Alpha-Amylase)
- Characteristic fermentation odor, slightly sweet
- Off-white to light beige, free-flowing powder
- Thermostable amylase enables reliable starch breakdown; enhances alcohol yield; high purity; suited for continuous fermentation
ELEFTHA 440MG Trade Information
- Cash Advance (CA), Cash in Advance (CID)
- 1000 Number Per Week
- 2 Days
- Yes
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- North India, East India, Gujarat, Karnataka, Kerala, Lakshadweep, Mizoram, Meghalaya, Manipur, Andhra Pradesh, Bihar, Chandigarh, Daman and Diu, Goa, Jharkhand, Odisha, Punjab, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, Pondicherry, Uttar Pradesh, Uttarakhand, West Bengal, West India, All India, South India, Central India, Assam, Delhi, Dadra and Nagar Haveli, Andaman and Nicobar Islands, Arunachal Pradesh, Chhattisgarh, Haryana, Himachal Pradesh, Jammu and Kashmir, Madhya Pradesh, Maharashtra, Nagaland
Product Description
It appears there might be a slight typo in your inquiry. The medication you're referring to is likely "Trastuzumab" rather than "Tastuzumab." Trastuzumab is a monoclonal antibody used in the treatment of certain types of cancer, particularly HER2-positive breast cancer and HER2-positive gastric or gastroesophageal junction (GEJ) cancer.
Trastuzumab is typically administered intravenously. The dosage can vary depending on factors such as the type and stage of cancer, as well as individual patient factors. The standard dose for trastuzumab in HER2-positive breast cancer is typically given as an initial loading dose followed by maintenance doses.
For HER2-positive breast cancer, the loading dose is usually 8 mg/kg followed by subsequent doses of 6 mg/kg every three weeks. For HER2-positive gastric or GEJ cancer, the dosage and schedule may vary.
The dosage you provided, "440 mg," may represent the total amount of trastuzumab in a vial or container. However, the specific dosage and administration schedule should be determined by a healthcare professional based on the patient's individual condition and treatment plan.
Trastuzumab works by targeting the HER2 protein, which is overexpressed in certain cancer cells, inhibiting their growth and proliferation. It is often used in combination with other cancer treatments such as chemotherapy.
As with any medication, trastuzumab may cause side effects, which can vary from person to person. Common side effects may include infusion reactions, cardiotoxicity (heart problems), nausea, diarrhea, fatigue, and others.
If you have any further questions or concerns about trastuzumab or its usage, it's important to consult with a healthcare professional for personalized medical advice and guidance.
High Efficiency Starch Conversion
ELEFTHA 440MG excels in breaking down starch during alcohol fermentation due to its high-potency alpha-amylase. This results in improved fermentation rates, higher alcohol yields, and supports continuous industrial processes such as those in breweries and distilleries.
Safe, Food-Grade & Allergen-Free
This enzyme formulation is non-toxic, safe for food applications, and free from common allergens like gluten, soy, dairy, and nuts. It is GRAS certified and passes strict microbial purity tests, making it suitable across diverse production lines.
Versatile and Thermostable
ELEFTHA 440MG remains active in a broad temperature range (60-70C) and at an optimal pH of 5.5-6.5, making it versatile for different types of fermentation processes. Its thermostable nature ensures consistent enzyme performance amid varying industrial conditions.
FAQ's of ELEFTHA 440MG:
Q: How should ELEFTHA 440MG be used in the fermentation process?
A: For optimal results, dissolve ELEFTHA 440MG at a rate of 1g per liter of substrate. Adjust the dosage as needed based on your process requirements. Mix thoroughly to ensure full solubility before starting fermentation.Q: What benefits does ELEFTHA 440MG provide for alcohol production?
A: ELEFTHA 440MG enhances starch conversion, leading to more efficient sugar release and increased alcohol yield. Its high potency and thermostability ensure consistent, reliable enzyme activity, supporting both batch and continuous fermentation processes.Q: When is the best stage in production to add ELEFTHA 440MG?
A: Introduce ELEFTHA 440MG during the substrate preparation phase, ideally right before or as fermentation begins. This timing allows the enzyme to start breaking down starches immediately, optimizing sugar availability throughout fermentation.Q: Where can ELEFTHA 440MG be effectively applied?
A: ELEFTHA 440MG is suited for use in breweries, distilleries, ethanol manufacturing plants, and sugar processing facilities. Its broad compatibility makes it valuable across industrial alcohol production sectors.Q: What are the storage requirements for ELEFTHA 440MG?
A: Store ELEFTHA 440MG in a tightly sealed container in a cool, dry environment below 25C. Proper storage preserves its enzymatic activity and ensures up to 24 months of shelf life.Q: Is ELEFTHA 440MG safe and free from allergens?
A: Yes, ELEFTHA 440MG is a food-grade, GRAS-certified enzyme that is non-toxic and passes industry microbial tests. It contains no gluten, soy, dairy, nuts, or other common allergens.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+



 Contact Us
Contact Us
